

3DMed Diagnostics Donates Medical Supplies to Support COVID-19 Fight in Cam3DMed Diagnostics - Approval for China's First ✱Fluorescent qPCR-based COVID19 plus Influenza A/B Combo Test Kit!
2021-8-16 16:00
Aug. 16, 2021 The “2019-nCoV and Influenza A/B Nucleic Acid Combo Test Kit (fluorescent PCR)” developed and manufactured by 3D Medicines Co., Ltd. (3Dmed) has been approved by the National Medical Products Administration (NMPA)!
The approval of this 3DMed product realizes the dual detection of influenza virus, a common pathogen in respiratory infections, and COVID19, the biggest global threat at the moment. A single test is capable of providing results for two major types of infectious pathogens, and the product has been verified to be compatible with several mainstream fluorescent quantitative PCR devices on the market (at present, clinical testing laboratories in China are mostly equipped with 4-plex or higher PCR machines). With this product, clinical physicians are able to effectively differentiate between novel coronavirus and influenza virus infections, thus helping them identify the cause of fever, select a reasonable diagnosis and treatment plan, and manage patients accordingly to prevent mass transmissions. Moving forward, we may continue to bear the strain of imported COVID19 cases and it will be increasingly important to be able to distinguish between and accurately diagnose influenza and novel coronavirus infection cases. SHANGHAI, Sept. 8, 2021 /PRNewswire/ -- Shanghai-based biotechnology company, 3DMed Diagnostics, has donated
anti-coronavirus medical appliances worth US$218,000 to Cambodia's Ministry of Health to support its campaign against COVID-19. A handover ceremony was held on Friday September 3 via video in Shanghai with Cambodian
officials participating from their home country.
Handover ceremony of Shanghai’s donation of medical supplies to Cambodia to combat COVID-19
The donation will help Cambodia better control COVID-19 with equipment and medical supplies for COVID-19 testing,
detection and diagnosis, including ANDiS 3100 Automated Sample Preparation Workstation, ANDiS 350 Automated Nucleic Acid Extraction System, ANDiS 300 Liquid Handling Workstation, Real-time Quantitative PCR Instrument,ANDiS Fast SARS-CoV-2 RT-qPCR Detection Kit, and Nucleic Acid Extraction Kit.
In collaboration with the Institut Pasteur du Cambodge (IPC), a Cambodian non-profit research institution, 3DMed aims to greatly improve the lab’s efficiency in testing and detecting COVID-19 in Cambodia. Compared with the 4–5-hour turnaround time of regular testing methods, the rapid test solutions by 3DMed can produce results in 70 minutes, which is crucial to conducting large-scale nucleic acid screening and testing across Cambodia.The donation also includes testing kits capable of detecting the delta variant and all other variants of interest (VOI) and variants of concern (VOC) identified by WHO, which will enable the health authority to accurately detect and control the spread of COVID variants.
With the recent surge of the delta variant, the Cambodian government is facing a great challenge to control the spread
of COVID-19. During the ceremony, Mr. Neang Samrith Komar, consul general of the Kingdom of Cambodia in Shanghai, conveyed his appreciation to China for its brotherly friendship and shared his belief that
Cambodia will win this fight against the pandemic.
Caifu Chen, Chief Technology Officer with 3D Med, said that since the start of the COVID-19 outbreak, the company has
continued to support more than 60 countries and regions in their battle against the pandemic. “We are proud to support the Cambodian people during this most difficult time, and we hope they can overcome the epidemic soon.”
About 3DMed Diagnostics:
Established in Shanghai in 2010,3DMed Diagnostics is one of the first companies to provide oncology precision medical
testing services in China. With long-term expertise in this field, 3DMed Diagnostics has become one of the market leaders in precision oncology diagnostics in China since its spin-off from the group company.

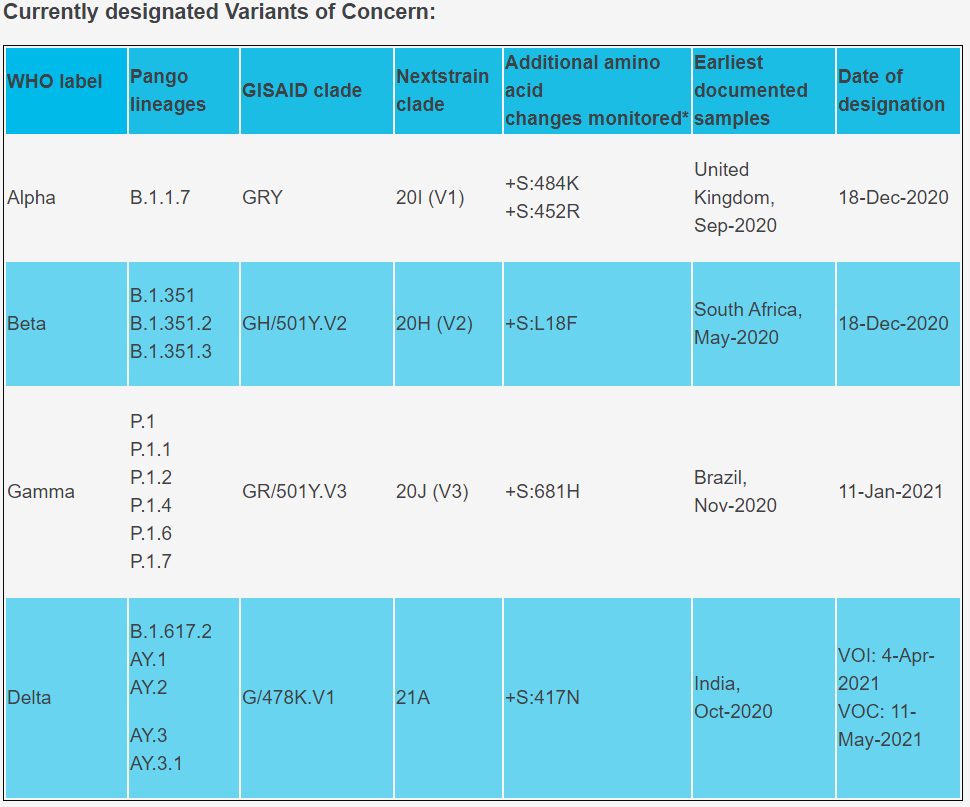
The test kit is able to detect the Delta strain and other variants of concern (VOC) listed by the WHO, through biometric analysis and wet experiments, overcoming loss of sensitivity due to mutations.

It is recommended to use the “2019-nCoV and Influenza A/B Nucleic Acid Combo Test Kit (fluorescent PCR)” developed by 3DMed Diagnostics together with its ANDiS 350, a fully automated nucleic acid extraction system and the corresponding reagents. The combo test kit is also compatible with other mainstream nucleic acid extraction systems that are currently available on the market.