

About us

Mission
To provide every patient access to precision treatment at any time and place.
Established in Shanghai in 2010, 3D Medicines (3DMed) is one of the first companies to provide oncology precision medical testing services in China. Over the years, the Company has grown into a successful Group consisting of 3DMed Diagnostics (Full company name: Etis Biotechnology (Shanghai) Co., Ltd.) and 3DMed (focusing on development of cancer drugs), with the former already well-established as a market leader in automated and smart precision diagnostics in China.
The Group has received more than CNY 4 billion in investments from renowned venture capital
firms, including more than CNY 20 billion for 3DMed Diagnostics. The Company has more than 1100 employees,
including more than 300 R&D personnel, and has applied/owns nearly 200 patents. Having built a
comprehensive R&D platform that integrates engineering, electrical, life science technologies, materials
science, and artificial intelligence data analysis, the Company is able to realize fully automated R&D
and manufacturing operations, as well as smart data processing, in the field of precision diagnosis.
In addition, the Company uses industry-leading third-party medical test centers with dual CAP/CLIA accreditation to offer precision diagnostic services to a network in which core AAA hospitals account for more than 70%). The Company works with more than 800 hospitals, to provide them with products and services that cover the entire process of precision medicine from early disease diagnosis, companion diagnostics to dynamic monitoring.
Guided by its “Patients First” motto, the Company stepped up and joined the fight against the pandemic immediately after the outbreak of the novel coronavirus in 2020. Due to its significant contributions, the Company, based in Minhang district was recognized as a “key organization in the fight against COVID19 pandemic (Shanghai)". At the same time, the Company has assisted in the global fight against the pandemic by developing related products and solutions such as novel coronavirus nucleic acid test kit, automated nucleic acid extraction kit, and mobile testing shelters. Its products and services are available in more than 60 countries and regions including Europe, U.S., Brazil, Australia, Middle East, Southeast Asia, and Africa.
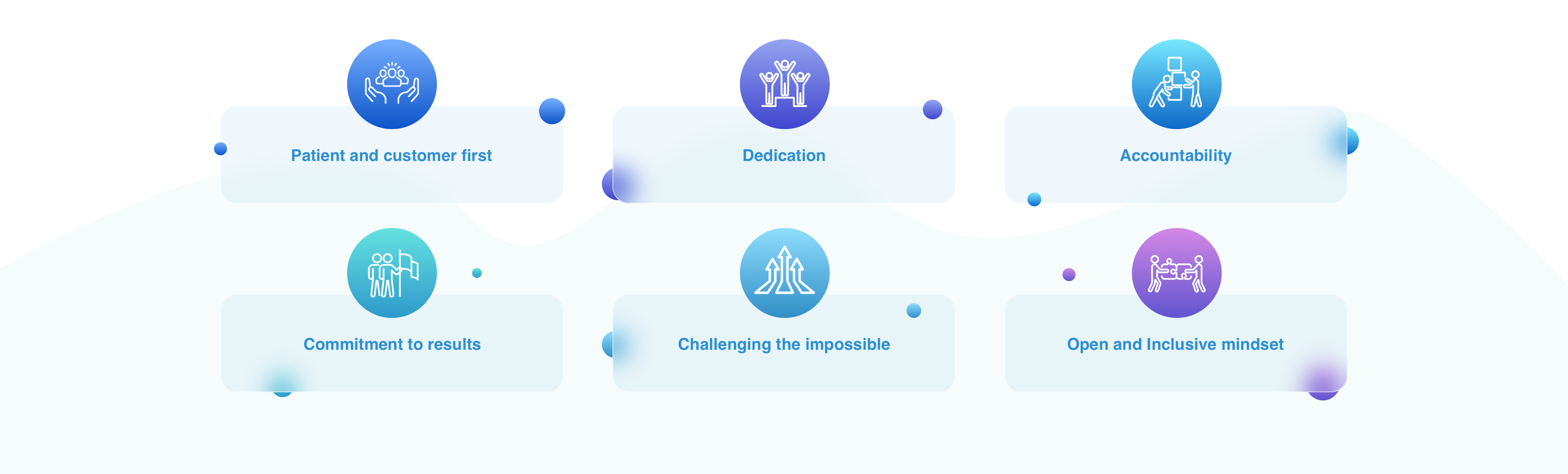
Culture & Values
Milestones
2021
A Class II Medical Device Registration Certificate was issued for ANDiS 500, which features automated closed preparation of gene libraries. This marked the first fully automated closed gene sequencing device to be approved in China.
The first COVID19 plus Influenza A/B combo detection kit based on fluorescent quantitative PCR platform in China to obtain marketing approval from the NMPA.
The Company’s medical laboratory was recognized a R&D institution in Pudong New Area.
Nominated as a “Certified Technical Center” in the Minhang district for 2021.
2020
The company made significant contributions in terms of nucleic acid testing in Wuhan during the pandemic and was recognized as a key organization in the fight against the COVID-19 pandemic (Shanghai).
2019
Recognized as a "National High-tech Enterprise"
Class II Medical Device Registration Certificate was issued for ANDiS 400, which features automated closed preparation of gene libraries.
Kicked off clinical study on early diagnosis of ovarian cancer
Released brand new flagship Panel GPS (Genomic Profiling System)
Third-party testing center has a CLIA-certified laboratory, and achieved the dual certification by CLIA and CAP.
Launched myTCGA database project targeting third-party testing services.
Developed TINA, a testing data management platform for hospital admission and IVD services.
Official split between 3DMed Diagnostics and 3DMed, with the former being the first to complete the corporate spin-off and launching the first round of independent fundraising.
2018
Released “non-invasive version” of OK companion which included top bTMB and bMSI technologies, hence realizing liquid biopsy in companion diagnostics for cancer immunotherapy.
Initiated critical Phase II trial for KN035 and China’s first marker-driven clinical study across different types of cancer.
Constructed industrial-grade biometric high-performance computing framework.
Realizes total automation of dry laboratory process
2017
Recognized as a "High-tech Enterprise" in Shanghai
Released “full version” of OK companion, a companion diagnostic product for cancer immunotherapy as a precision medicine treatment.
KN035 was approved for clinical studies in Japan (the first new-generation anti-PD-L1 single-domain antibody drug to commence simultaneous clinical phase in China, the United States, and Japan).
Constructed an integrated data platform that supports diagnostic services
2016
Released the Luming series (tissues), Dike series (ctDNA), and Zhian series (germ line) as featured precision diagnostic products in cancer treatment.
KN035 was approved by the U.S. FDA for clinical studies in the United States.
KN035 was approved by the CFDA for clinical studies in China
2015
Offered precision diagnosis services in more than 200 AAA hospitals across the country
2014
Developed personalized precision diagnosis for cancer patients
2013
Constructed the lentiviral ShRNA library with more than 3000 tumor-related genes
Constructed the world's largest marker development platform for precision medicine targeting liver cancer
2012
Completed initial round of funding
Constructed marker development platform for tumor-targeted drugs
2011
Constructed the first gene function/drug target research platform based on RNAI library in China.
2010
Incorporated in Shanghai on Dec. 27, 2010.
Add : Building 2B2F, 158 XinJunhuan Street, Pujiang Hi-tech Park, Shanghai, China, 201114